YouTHrive was a two-arm randomized controlled trial (RCT) to test the efficacy of an adapted version of the Thrive with Me intervention for youth living with HIV (YLWH).
Protocol Overview
YouTHrive is a 2-arm prospective randomized controlled trial (RCT) in Atlanta, Chapel Hill, Charlotte, Chicago, Houston, New York City, Philadelphia, and Tampa. To inform intervention development, we conducted 6 focus groups in Chicago, Houston, and New York and usability testing at all recruitment sites.
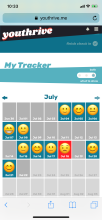
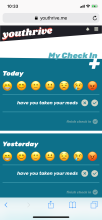
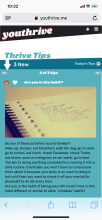
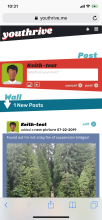
Youth randomized to the intervention condition have access to a mobile-optimized website for 5 months that has the following features: 1) a community wall where youth can interact with each other by posting comments and replying to other participant’s comments; 2) daily rotating informational, motivational, and behavioral skills tips about living with HIV; 3) self-monitoring of HIV medications and mood; 4) and goal setting and goal tracking a suggested or a custom goal. As youth interact with the site, they will collect points and move up levels. With each new level, new features of the site are unlocked such as new avatars and color theme options.
Participants assigned to the control condition receive a weekly email newsletter for 5 months with content about living with HIV and general well-being.
Principal Investigators:
Keith Horvath, PhD | San Diego State University
K. Rivet Amico, PhD | University of Michigan
ClinicalTrials.gov Number: NCT03149757
Duration
Participants in both the intervention and control arms are enrolled for either 5 or 11 months.
Sample Size
Total study sample is 368 of which the following will be enrolled for each phase: 1) Up to 48 participants for focus groups to inform intervention adaption; 2) 12-20 participants for usability testing to finalize intervention components; 3) 300 participants for a randomized controlled trial of YouTHrive, with participants randomized to either YouThrive (n=150) or control (n=150).
Eligibility
- 15-24 years of age at the enrollment visit
- Living with HIV
- Residing in Atlanta, Chapel Hill, Charlotte, Chicago, Houston, New York City, Philadelphia, or Tampa area and available to meet with SRV staff for visits either in-person or remotely at baseline, and 5-month and 11-month follow-up assessments. Some participants will receive a shortened intervention without the 11-month follow-up assessment.
- Evidence of an ART prescription with refills
- Anticipated continuous internet access and SMS messaging for the intervention period (approximately 5 months)
- Meets medical-chart verified or self-reported criteria
- Full list of eligibility and exclusion criteria available on ClinicalTrials.gov
Outcomes
Primary Objective: In a 2-arm RCT (n=300), assess the efficacy of YouTHrive to sustain suppressed viral load among youth living with HIV, compared to an HIV information-only control condition.
Secondary Objective: Assess whether YouTHrive is more beneficial for substance-using than non substance-using youth living with HIV.
Publications
- Hightow-Weidman LB, Horvath KJ, Scott H, Hill-Rorie J, Bauermeister JA. Engaging youth in mHealth: what works and how can we be sure? mHealth. 2021;7:23. doi: 10.21037/mhealth-20-48. eCollection 2021. PubMed PMID: 33898592; PubMed Central PMCID: PMC8063019.
- Horvath KJ, Amico KR, Erickson D, Ecklund AM, Martinka A, DeWitt J, McLaughlin J, Parsons JT. Thrive With Me: Protocol for a Randomized Controlled Trial to Test a Peer Support Intervention to Improve Antiretroviral Therapy Adherence Among Men Who Have Sex With Men. JMIR Res Protoc 2018;7(5):e10182. DOI: 10.2196/10182. PMID: 29853437
- Muessig KE, LeGrand S, Horvath KJ, Bauermeister JA, Hightow-Weidman LB. (2017). Recent mobile health interventions to support medication adherence among HIV-positive MSM. Curr HIV/AIDS Rep. 2017 Sep;12(5):432-441. doi: 10.1097/COH.0000000000000401. Review. PubMed PMID: 28639990; PubMed Central PMCID: PMC5762120.
- Horvath, K., Lammert, S., LeGrand, S., Muessig, K., Bauermeister, J. Using technology to assess and intervene with illicit drug-using persons at risk for HIV. Curr Opin HIV AIDS. 2017 Sept.
- Bauermeister JA, Golinkoff JM, Muessig KE, Horvath KJ, Hightow-Weidman LB. (2017). Addressing engagement in technology-based behavioural HIV interventions through paradata metrics. Curr Opin HIV AIDS. 2017 July.