A novel mobile app to support linkage to HIV/STI testing and PrEP for young men who have sex with men
Protocol Overview
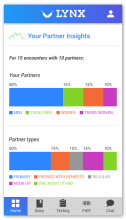
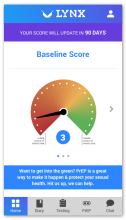
Using the Information-Motivation-Behavior Skills (IMB) model, we have developed LYNX, a highly interactive mobile app to promote accurate risk perception and increase HIV/STI testing and linkage to care among young men who have sex with men (YMSM). Key components of the app include Sex Pro (a personalized HIV risk score), a sex diary to facilitate accurate data collection; HIV/STI testing information and reminders; access to home HIV/STI testing options; a geospatial-based locator of HIV/STI testing sites and PrEP clinics; PrEP information and videos; and online PrEP navigation. Features of the app will be finalized during the formative phase of this study.
The LYNX protocol involved qualitative formative work through focus groups, to refine the LYNX app (Phase 1); an open technical pilot to optimize usability (Phase 2); and a pilot randomized controlled trial (RCT) to evaluate preliminary acceptability, feasibility, and preliminary efficacy (Phase 3) of the LYNX app.
Principal Investigators:
Albert Liu, MD, MPH | San Francisco Department of Public Health
Hyman Scott, MD, MPH | San Francisco Department of Public Health
ClinicalTrials.gov Number: NCT03177512
Duration
Formative work (Aim 1) was conducted over 6 months. The technical pilot (Aim 2) enrolled over a 2-month period, and study follow-up will be 2 months. The pilot RCT (Aim 3) was enrolled over several months, and study follow-up was 6 months.
Sample Size
Total sample size for this project was up to 115 YMSM. Aim 1 included 40 young men who have sex with men (YMSM) and who are not living with HIV. Aim 2 included up to 15 YMSM. Aim 3 enrolled 60 YMSM, with 40 YMSM randomized to receive LYNX, and 20 YMSM randomized to receive standard or care.
Eligibility
- Age 15-24 years old
- Assigned male sex at birth and male-identified
- Self-report being HIV uninfected or HIV status unknown at screening
- Self-report having not had an HIV test in the past 3 months (for Aims 2 and 3 only)
- Owns an iOS or Android mobile phone
- Full list of eligibility and exclusion criteria available on ClinicalTrials.gov
Outcomes
Primary:
- To conduct qualitative, formative work to refine a mobile phone app, LYNX, to promote HIV/STI testing and PrEP uptake among YMSM.
- To conduct a technical pilot to optimize LYNX app functionality, technical performance, and user satisfaction.
- To conduct a pilot RCT to evaluate the feasibility and acceptability of LYNX to increase HIV/STI testing and PrEP uptake among YMSM.
Secondary:
- To compare the rate of HIV/STI testing in the LYNX intervention vs. control arms.
- To compare the rate of PrEP uptake in the LYNX intervention vs. control arms.
- To compare changes in sexual risk behaviors among YMSM in the LYNX intervention vs. control arms.
Publications
- Biello KB, Horvitz C, Mullin S, Mayer KH, Scott H, Coleman K, Dormitzer J, Norelli J, Hightow-Weidman L, Sullivan P, Mimiaga MJ, Buchbinder S, Bojan K, Futterman D, Emmanuel P, Liu A. HIV self-testing and STI self-collection via mobile apps: experiences from two pilot randomized controlled trials of young men who have sex with men. mHealth. 2021;7:26. doi: 10.21037/mhealth-20-70. eCollection 2021. PubMed PMID: 33898595; PubMed Central PMCID: PMC8063023.
- Giovenco D, Muessig KE, Horvitz C, Biello KB, Liu AY, Horvath KJ, Golinkoff JM, Reback CJ, Hightow-Weidman L. Adapting technology-based HIV prevention and care interventions for youth: lessons learned across five U.S. Adolescent Trials Network studies. mHealth. 2021;7:21. doi: 10.21037/mhealth-20-43. eCollection 2021. PubMed PMID: 33898590; PubMed Central PMCID: PMC8063021.
- Liu A, Coleman K, Bojan K, Alonso Serrano P, Oyedele T, Garcia A, Enriquez-Bruce E, Emmanuel P, Jones J, Sullivan P, Hightow-Weidman L, Buchbinder S, Scott H. Developing a Mobile App (LYNX to Support Linkage to HIV/Sexually Transmitted Infection Testing and Pre-Exposure Prophylaxis for Young Men Who Have Sex With Men: Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2019 (Jan 25); 8(1):e10659.