Mobile-based application to increase uptake of HIV testing, detection of new HIV infections, and linkage to care and prevention services by young men who have sex with men
Protocol Overview
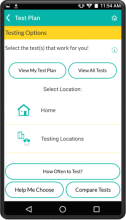
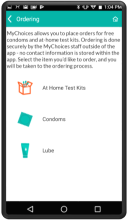
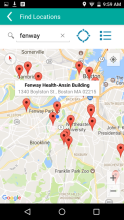
MyChoices is an intervention development, feasibility and acceptability study of a theory-driven mobile app to increase HIV testing and PrEP uptake by young men who have sex with men (YMSM), ages 15-24. MyChoices app was refined in theater testing and an open technical pilot, and then the acceptability and feasibility of this integrated app was evaluated in a pilot randomized controlled trial (RCT) among YMSM at risk for HIV acquisition in the US.
Principal Investigators:
Katie Biello, PhD, MPH | Center for Health Equity Research (CHER)
Kenneth Mayer, MD | The Fenway Institute
ClinicalTrials.gov Number: NCT03179319
Duration
Aim 1: single study visit (focus group) for theater testing
Aim 2: 2 months
Aim 3: 6 months
Sample Size
Aim 1: Up to 40 YMSM
Aim 2: Up to 15 YMSM across two iTech SRVs
Aim 3: 60 YMSM across four cities: Atlanta, Boston, Chapel Hill, and New York City
Eligibility
- Between 15 and 24 years of age
- Assigned male sex at birth and male-identified
- Self-report being HIV uninfected or HIV status-unknown at screening
- Self-report having not had an HIV test in the past 3 months (for Aims 2 and 3 only)
- Not currently on PrEP (for Aims 2 and 3 only)
- Able to understand, read, and speak English
- Owns or leases a phone with Android platform (for Aims 2 and 3) or iOS platform (Aim 3 only), and has an active data plan
- Full list of eligibility and exclusion criteria available on ClinicalTrials.gov
Outcomes
- To refine a mobile phone app, “MyChoices”, to promote HIV/STI testing and PrEP uptake among YMSM by conducting theater testing with up to 20 YMSM. Data will be used to make final app refinements.
- To conduct a technical pilot with up to 15 YMSM to optimize MyChoices app functionality, technical performance, and user satisfaction. Data will be used to finalize the pilot study methods, the mobile app functionalities, and measures for the pilot trial (Aim 3).
- To conduct a pilot randomized controlled trial (RCT) with up to 60 YMSM to evaluate the feasibility and acceptability of MyChoices to increase HIV/STI testing and PrEP uptake among YMSM. Every 3 months, participants will complete a brief quantitative assessment (in person at baseline and online at follow-up) and app usage patterns will be assessed. The primary outcomes will be acceptability (mean score on the System Usability Scale) and feasibility (participants utilizing the app at least once during follow-up) of the app.
Publications
- Biello KB, Hill-Rorie J, Valente PK, Futterman D, Sullivan PS, Hightow-Weidman L, Muessig K, Dormitzer J, Mimiaga MJ, Mayer KH. Development and Evaluation of a Mobile App Designed to Increase HIV Testing and Pre-exposure Prophylaxis Use Among Young Men Who Have Sex With Men in the United States: Open Pilot Trial. J Med Internet Res. 2021 Mar 24;23(3):e25107. doi: 10.2196/25107. PubMed PMID: 33759792.
- Biello KB, Horvitz C, Mullin S, Mayer KH, Scott H, Coleman K, Dormitzer J, Norelli J, Hightow-Weidman L, Sullivan P, Mimiaga MJ, Buchbinder S, Bojan K, Futterman D, Emmanuel P, Liu A. HIV self-testing and STI self-collection via mobile apps: experiences from two pilot randomized controlled trials of young men who have sex with men. mHealth. 2021;7:26. doi: 10.21037/mhealth-20-70. eCollection 2021. PubMed PMID: 33898595; PubMed Central PMCID: PMC8063023.
- Giovenco D, Muessig KE, Horvitz C, Biello KB, Liu AY, Horvath KJ, Golinkoff JM, Reback CJ, Hightow-Weidman L. Adapting technology-based HIV prevention and care interventions for youth: lessons learned across five U.S. Adolescent Trials Network studies. mHealth. 2021;7:21. doi: 10.21037/mhealth-20-43. eCollection 2021. PubMed PMID: 33898590; PubMed Central PMCID: PMC8063021.
- Biello KB, Marrow E, Mimiaga MJ, Sullivan P, Hightow-Weidman L, Mayer KH. A Mobile-Based App (MyChoices) to Increase Uptake of HIV Testing and Pre-Exposure Prophylaxis by Young Men Who Have Sex With Men: Protocol for a Pilot Randomized Controlled Trial. JMIR Res Protoc 2019;8(1):e10694. DOI: 10.2196/10694 PMID: 30617042
- Liu A, Biello K, Zlotorzynska M. HIV self-testing and STI self-collection via mobile apps: experiences from two pilot randomized controlled trials of young men who have sex with men. mHealth. July 15, 2020. doi: 10.21037/mhealth-20-70